Independent work:
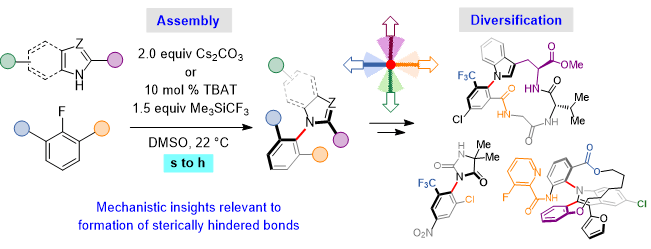
15. Nucleophilic Aromatic Substitutions Enable Diversity-Oriented Synthesis of Heterocyclic Atropisomers via Non-Atropisomeric Intermediates
M. Šimek,# P. Dey,# V. Blahout, K. Mondal, J. Ernenwein, M. Dračínský, D. Bím,* P. H. S. Paioti,*
Nat. Commun. 2025, 16, 4856. (#equal contribution)
https://www.nature.com/articles/s41467-025-60101-z
—————————————————————————————
Mentored work:
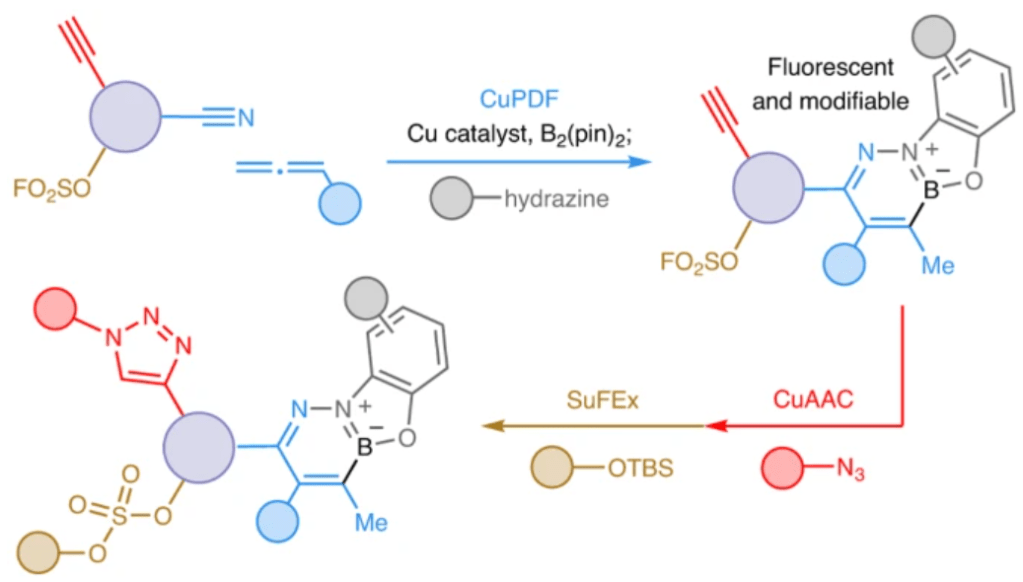
14. Click Processes Orthogonal to CuAAC and SuFEx Forge Selectively Modifiable Fluorescent Linkers
P. H. S. Paioti, K. E. Lounsbury, F. Romiti, M. Formica, V. Bauer, C. Zandonella, M. E. Hackey, J. del Pozo, A. H. Hoveyda*
Nat. Chem. 2024,16, 426–436.
https://www.nature.com/articles/s41557-023-01386-9
—————————————————————————————
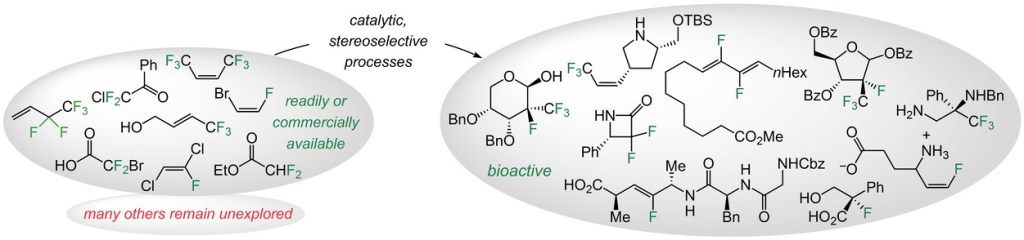
13. Catalytic and Stereoselective Transformations with Easily Accessible and Purchasable Allyl and Alkenyl Fluorides
P. H. S. Paioti,* S. A. Gonsales,* S. Xu., A. Nikbakht, D. C. Fager, Q. Liu, A. H. Hoveyda*
Angew. Chem. Int. Ed. 2022, 61, e202208742 (Review Article)
https://onlinelibrary.wiley.com/doi/full/10.1002/ange.202208742
—————————————————————————————
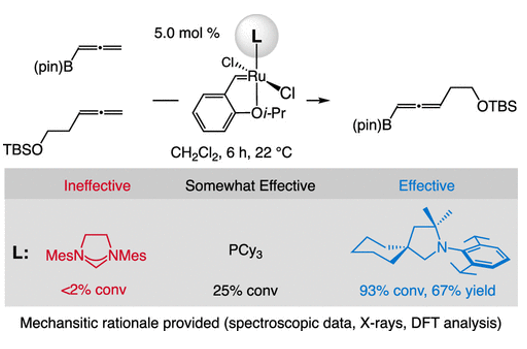
12. Cross-metathesis of Allenes. Mechanistic Analysis and Identification of a Ru-CAAC as the Most Effective Catalyst
S. A. Gonsales, Z. C. Mueller, F. Zhao, P. H. S. Paioti, L. Karmazin, J. Wan, F. Liu,* K. N. Houk,* A. H. Hoveyda*
J. Am. Chem. Soc. 2021, 143, 20640–20644.
https://pubs.acs.org/doi/full/10.1021/jacs.1c11453
—————————————————————————————
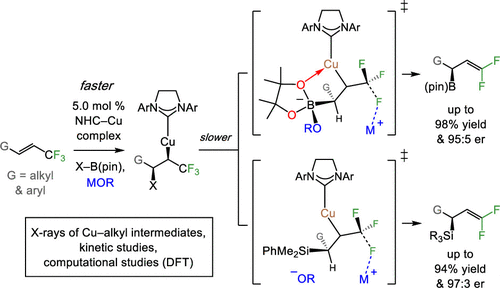
11. Catalytic Enantioselective Boryl and Silyl Substitutition with Trifluoromethyl Alkenes: Scope, Utility, and Mechanistic Nuances of Cu–F beta-Elimination
P. H. S. Paioti,# J. del Pozo,# M. S. Mikus,# J. Lee, M. J. Koh, F. Romiti, S. Torker,* A. H. Hoveyda*
J. Am. Chem. Soc. 2019, 141, 19917–19934. (#equal contribution)
https://pubs.acs.org/doi/full/10.1021/jacs.9b11382
—————————————————————————————
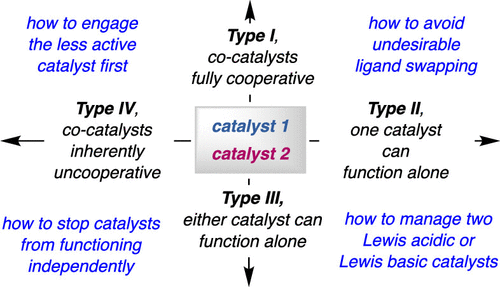
10. Different Strategies for Designing Dual-Catalytic Enantioselective Processes: from Fully Cooperative to Non-Cooperative Systems
F. Romiti, J. del Pozo, P. H. S. Paioti, S. A. Gonsales, X. Li, F. W. W. Hartrampf, A. H. Hoveyda*
J. Am. Chem. Soc. 2019, 141, 17952–17961. (Perspective Article)
https://pubs.acs.org/doi/10.1021/jacs.9b05464
—————————————————————————————
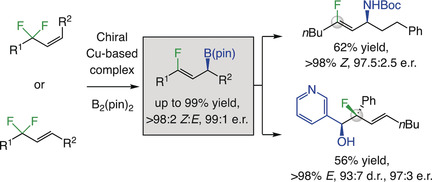
9. Catalytic Enantioselective Synthesis of Allylic Boronates bearing a Trisubstituted Alkenyl Fluoride and Related Derivatives
S. Akiyama, K. Kubota, M. S. Mikus, P. H. S. Paioti, F. Romiti, Q. Liu, Y. Zhou, A. H. Hoveyda,* H. Ito*
Angew. Chem. Int. Ed. 2019, 58, 11998–12003.
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201906283
—————————————————————————————
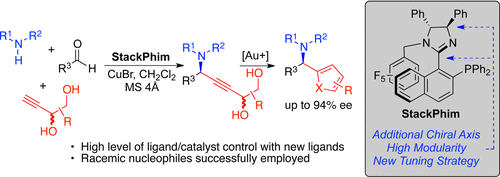
8. Incorporation of Axial Chirality into Phosphino-Imidazoline Ligands for Enantioselective Catalysis
P. H. S. Paioti, K. A. Abboud, A. Aponick*
ACS Catal. 2017, 7, 2133–2138.
https://pubs.acs.org/doi/full/10.1021/acscatal.7b00133
—————————————————————————————
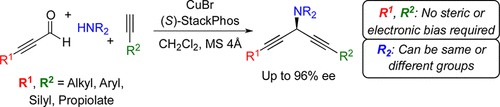
7. Catalytic Enantioselective Synthesis of Amino Skipped Diynes
P. H. S. Paioti, K. A. Abboud, A. Aponick*
J. Am. Chem. Soc. 2016, 138, 2150–2153.
https://pubs.acs.org/doi/full/10.1021/jacs.5b13387
—————————————————————————————
6. Gold-Catalyzed Transformation of Unsaturated Alcohols
P. H. S. Paioti, A. Aponick*
Topics in Current Chemistry – Springer 2015, 357, 63–94. (Book Chapter)
https://link.springer.com/chapter/10.1007/128_2014_590

—————————————————————————————
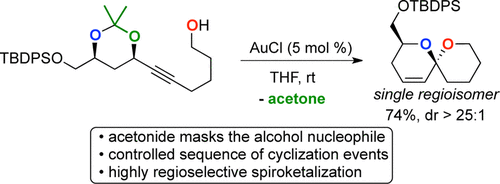
5. Controlling Regiochemistry in the Gold-Catalyzed Synthesis of Unsaturated Spiroketals
P. H. S. Paioti, John M. Ketcham, A. Aponick*
Org. Lett. 2014, 16, 5320–5023.
https://pubs.acs.org/doi/full/10.1021/ol5024954
—————————————————————————————
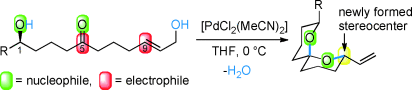
4. Pd(II)-Catalyzed Spiroketalization of Ketoallylic Diols
J. A. Palmes, P. H. S. Paioti, L. P. De Souza, A. Aponick*
Chem–Eur. J. 2013, 19, 11613–11621.
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.201301723
———————————————————————————————-
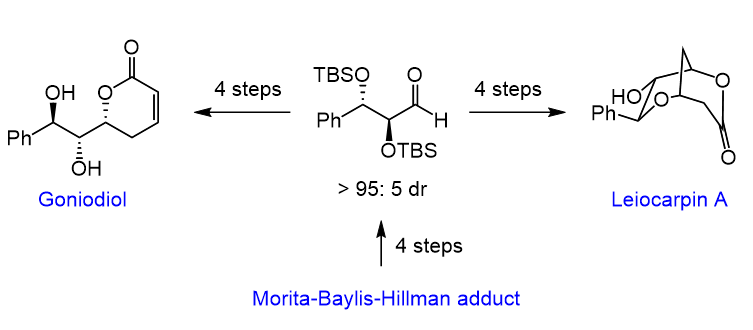
3. A Morita–Baylis–Hillman Adduct Allows the Diastereoselective Synthesis of Styryl Lactones
P. H. S. Paioti, F. Coelho*
Tetrahedron Lett. 2011, 52, 6180–6184.
https://www.sciencedirect.com/science/article/pii/S0040403911015656
———————————————————————————————-
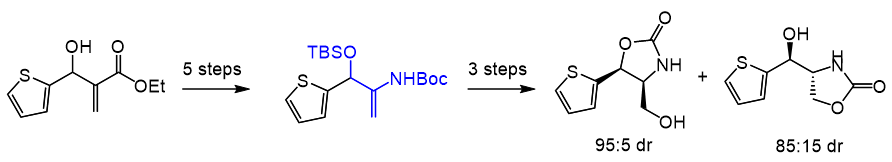
2. Diastereoselective Synthesis of Substituted 2-Amino-1,3-Propanediols from Morita‑Baylis-Hillman Adducts
P. H. S. Paioti, P. Rezende, F. Coelho*
J. Braz. Chem. Soc. 2012, 23, 285–293.
https://www.scielo.br/j/jbchs/a/mLYrSzPLJBb3wVnwMRgjz5c/abstract/?lang=en
———————————————————————————————-
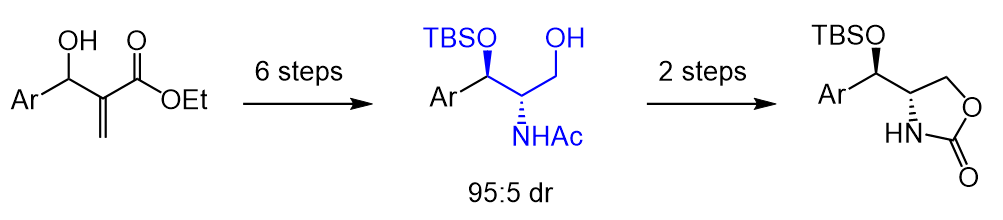
1. Diastereoselective Approach to Substituted Oxazolidinones from Morita-Baylis-Hillman Adducts
P. Rezende, P. H. S. Paioti, F. Coelho*
Synth. Commun. 2010, 41, 227–242.
https://www.tandfonline.com/doi/abs/10.1080/00397910903534023

“The most fundamental and lasting objective of synthesis is not production of new compounds, but production of properties.”
George S. Hammond

Institute of Organic Chemistry and Biochemistry – IOCB Prague
Address: Flemingovo nám. 542, 160 00 Praha 6-Dejvice
Homepage: https://www.uochb.cz/en
